Biomedical Optics Laboratory
ECU Biomedical Optics Laboratory includes a bio-optics lab (room E212), a photonics lab (E211a), a microbiology lab (room E217) and a machine/electronics lab (room E211b) located in the Howell Science Complex building. The optics laboratory is well equipped with three optical tables, lasers/optics, a diamond-anvil cell for high pressure study, and houses three modified research DIC/phase-contrast/fluorescence microscopes, two bench laser Tweezers and Raman spectroscopy (LTRS) setups, and a Raman and fluorescence imaging setup for the single cell studies. The microbiology lab is equipped for spore/bacteria preparation, storage, and treatment. The machine/electronics lab is equipped for making mechanic and electronic parts.
The major equipment includes three inverted DIC and phase-contrast microscope (Nikon TE2000 and TiS, Olympus IX81), atomic force microscope (easyScan 2, Nanosurf), two spectrometers (Jobin Yvon Triax320), two TE-cooled CCD detector (Princeton Instruments, PIXIS), two EMCCD (Andor DU-897), and two near-infared diode lasers (785nm/1500mW), two ultrafast pulsed lasers (Coherent 900-D and 900-P) for Raman microscopy and multi-photon microscopy, two solid-state green lasers (Coherent Verdi-10) used for the excitation source and optical pulling and two single photon detectors. The lab is also equipped with a XY motorized stage and vortex phase plate for the solid-phase Raman cytometry.
The capabilities include Laser tweezers and Raman spectroscopy system of biological particles suspending in liquid and in air with a size ranging from 0.2 to 100 microns; Confocal micro-Raman spectroscopy and Raman imaging for biological and chemical analysis; Differential interference contrast/fluorescence microscopes; Atomic force microscope; Automated imaging spectrometer for laser Raman spectroscopy from 200 to 2000 nm; Ultrafast pulsed laser sources tunable from 700 to 980 nm, pulse width of 100 fs or 1 ps, power up to 1.0 W at a repetition rate of 76 MHz, synchronized repetition. Pulse picker with EOM selects femto or pico second pulses with a rate from 1.0 kHz to 250 kHz. Electronic multiplying CCD with single photon counting capability; high power laser scissor (up to 1.0 J per pulse).
The research goal of our lab is to apply the principles and techniques of quantum optics for the detection, identification, and manipulation of fundamental biological processes at the level of single cells and single molecules. The current research includes Raman tweezers, optical pulling and trapping of airborne particles, and monitoring dynamic biological process of single living cells.
Optical Pulling of Airborne Particles over 10 Meters

Pulling and Lifting Macroscopic Objects by Light

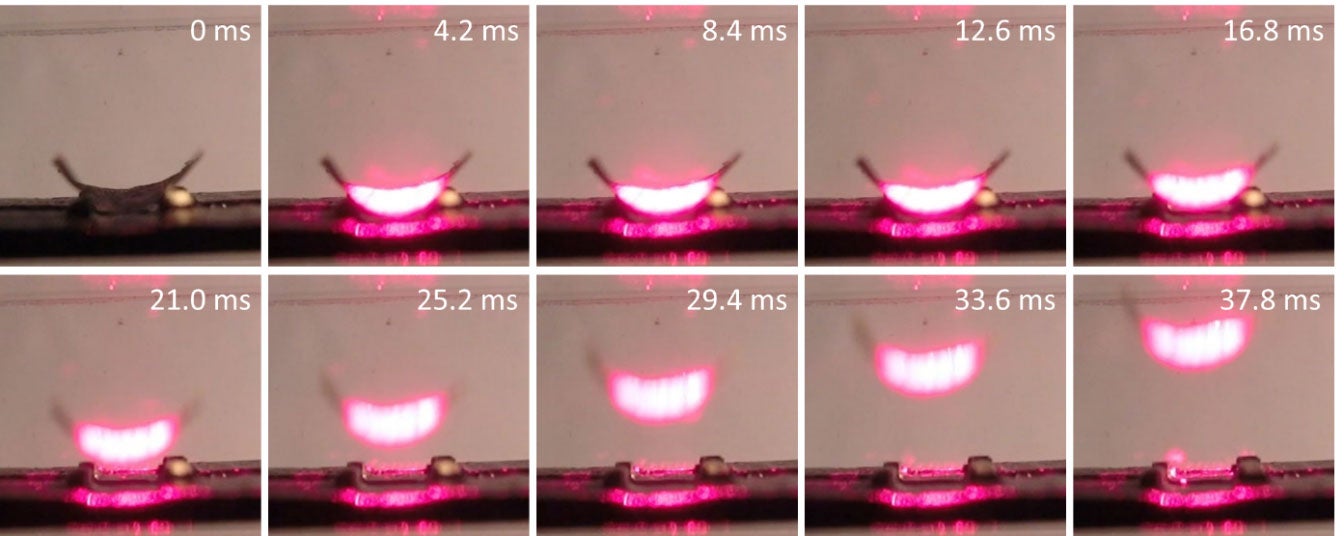
The lifting of a gold cylindrical vane (7×7 mm) by a 650 nm laser beam with a power of 1 W.
Raman Tweezers
It is the combination of Nobel-Prize winning optical tweezers and Raman spectroscopy, which allows capturing and manipulating a single biological particle including cell, bacterium and virus and allows acquiring the Raman spectroscopy of the trapped particles. Raman tweezers can provide biochemical composition of a single living cell without chemically interfering it and the measured vibrational energy levels can be used as fingerprints for identification of biological cells.

Biomedical optics laboratory.

Raman tweezers laboratory.
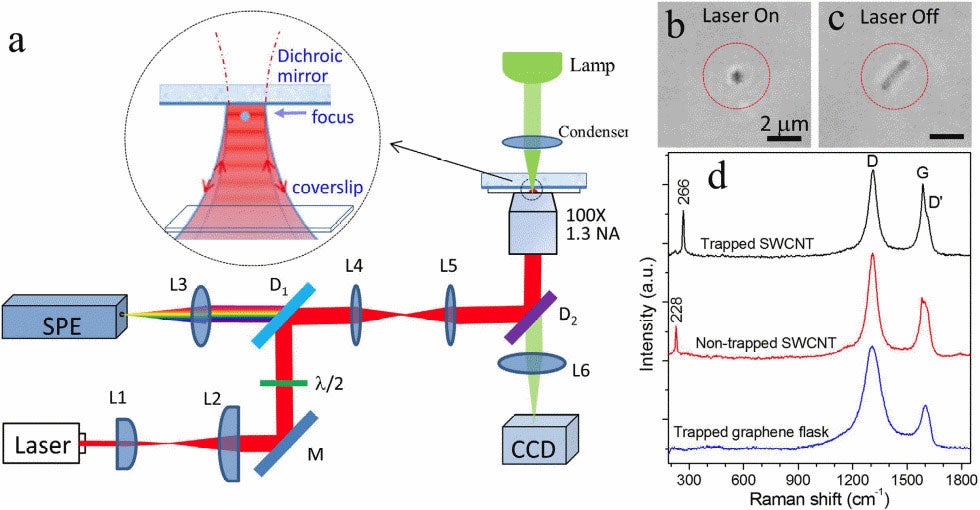
Raman tweezers is used for the stable optical trapping and characterization of nanoparticles and single cells. This figure’s source is part of a Nature collection on optical tweezers to celebrate the 2018 Nobel Prize in Physics.
Monitor Dynamic Biological Process of Single Cells and Cellular Heterogeneity by Live-Cell Microscopy and Spectroscopy

Picosecond and femtosecond pulsed lasers for ultrafast spectroscopy of single cells.
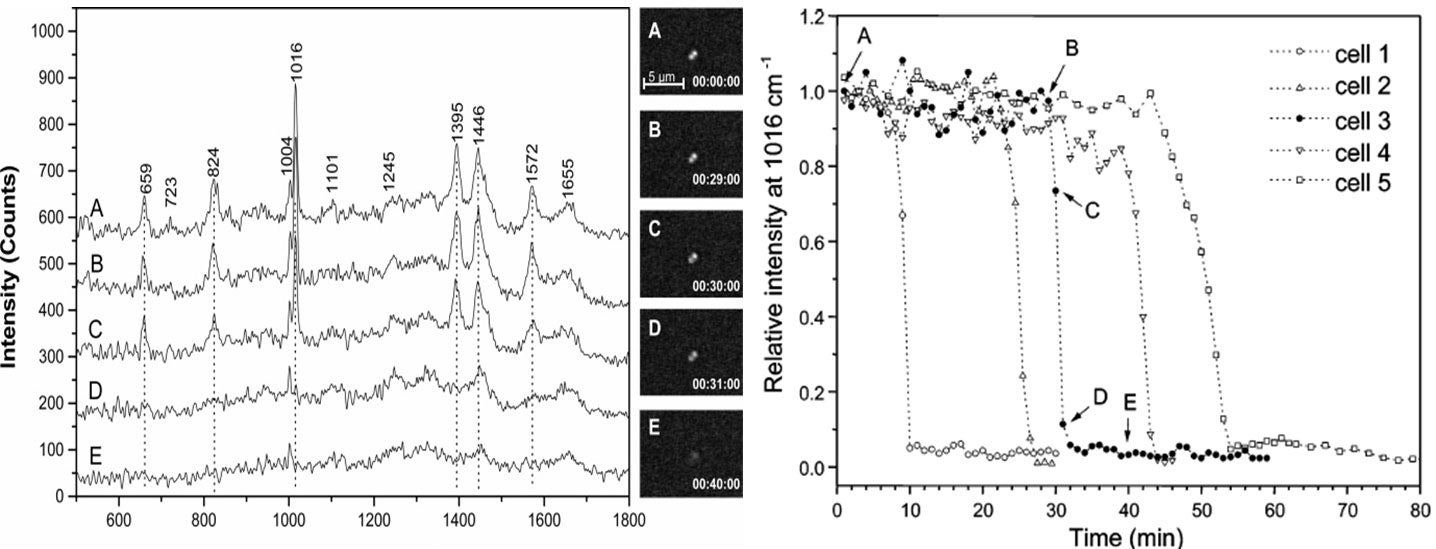
(left, above) Time-lapse Raman spectra of a single trapped Bacillus thuringiensis spore after exposure to the TSB growth medium and the corresponding DIC images (A, below). Curve A is for 0 min from the time when the spore was captured in the optical trap; B for 29 min; C for 30 min; D for 31min; and E for 40 min. (right, above) Intensities of the 1016 cm-1 CaDPA band of five individual Bt spores as a function of the incubation time.
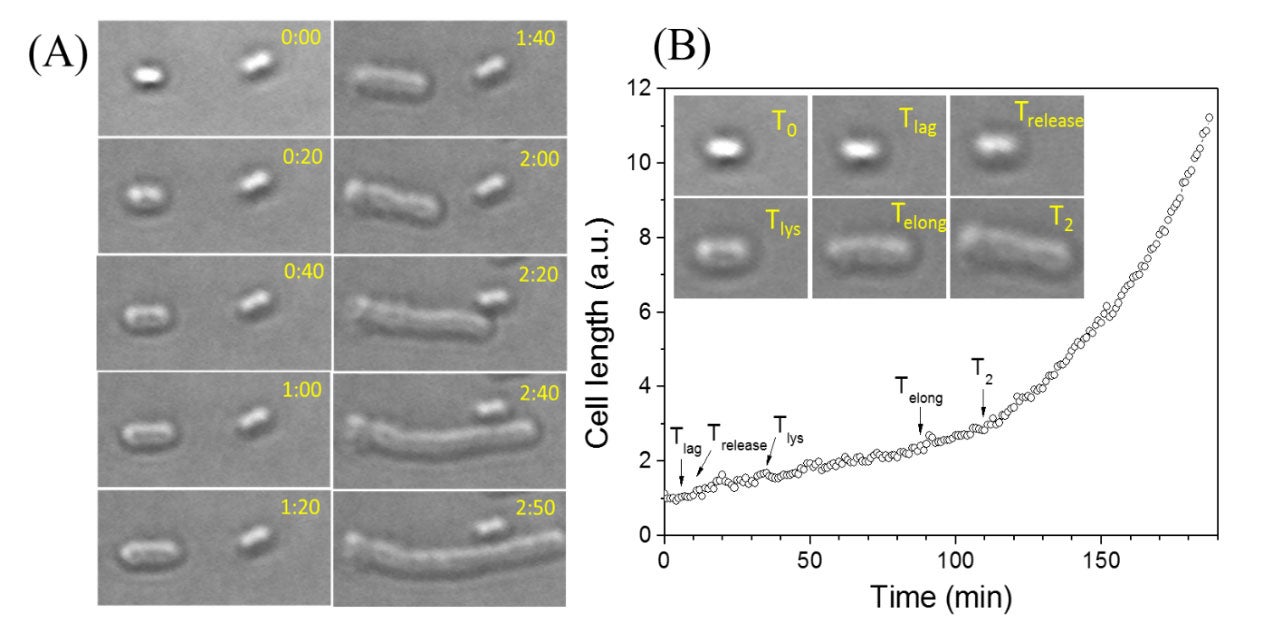
Live-cell optical microscopy monitors germination, outgrowth, and growth of single bacterial spores.