Biophysics / Bioacoustics
Nathan Hudson studies the exciting field of molecular biophysics. Work in his laboratory uses techniques such as protein engineering, centrifuge force microscopy, FRET, and microfluidics to understand how mechanical force regulates biological function. Projects involve measuring the biomechanical properties of blood coagulation proteins and determining the force-depending binding kinetics of adhesion molecules. There are both graduate- and undergraduate-level projects in the lab, and numerous students have won awards for their research with Dr. Hudson.

The fibrin molecule is the mechanical backbone of blood clots.
Xin-Hua Hu investigates the optical properties of biological samples and develops innovative instrumentation for the characterization of cells and turbid samples. Over the past decades he and Dr. Jun Q. Lu have developed in the Biomedical Laser Laboratory a laser-optics experimental research facility as well as a high-performance computing facility. Experimental measurements are carried out in the laser lab with in-house developed diffraction imaging flow cytometer, multispectral reflectance imaging systems, and multi-parameter spectrophotometry systems for image and spectroscopic data acquisition and analysis. Image processing and large scale numerical simulations have been actively pursued to clearly understand the correlations between cellular and turbid samples’ optical and morphological properties by imaging and measurement of scattered light signals. These research activities allow him to develop innovative approaches of acquiring big data on human cells and tissues with state-of-art machine learning algorithms and instrumentation for wide-ranged applications.
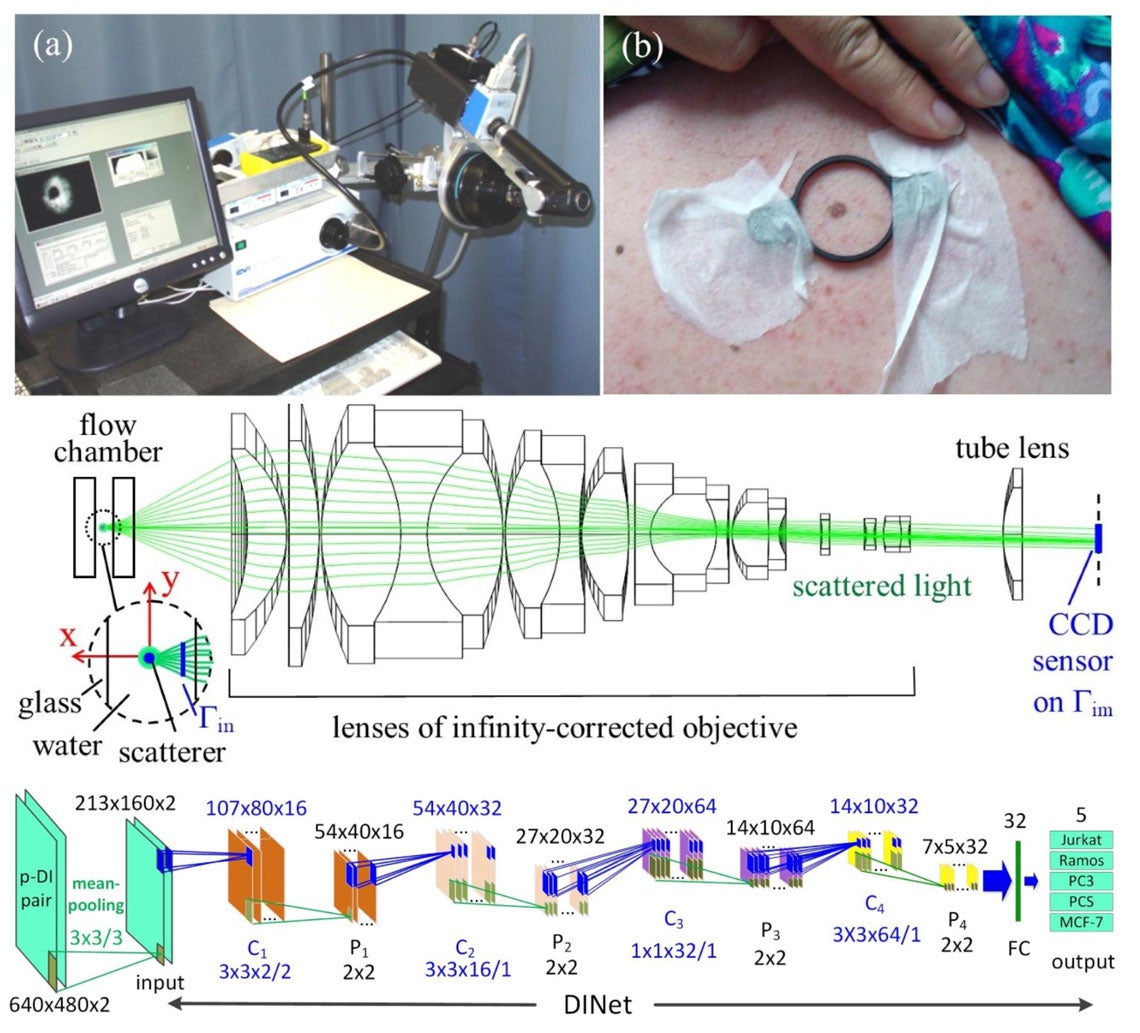
(a) A multispectral imaging system for multispectral imaging and diagnosis of cutaneous melanoma. (b) Preparation for image acquisition from an enrolled patient before surgical removal of a mole suspected of melanoma. (middle) The schematic of optical setup for diffraction imaging flow cytometry system with a microscope objective based imaging unit. (bottom) The dataflow graph of a deep learning neural network (DINet) developed for classification of 5 cell types based on cross-polarized diffraction image (p-DI) pair data.
Yong-Qing Li studies the exciting field of biophotonics. Work in the Biomedical Optics Laboratory uses techniques such as optical tweezers and Raman spectroscopy and imaging, live-cell light microscopy, and atomic force microscopy to understand the fundamental biological processes of single cells and cellular heterogeneity. Projects include optical pulling of airborne particles and lifting of large objects by light, monitoring dynamic germination, outgrowth, and growth of single bacterial spores in nutrients and high pressure environment, and label-free surface enhanced Raman spectroscopy (SERS) for Ras proteins-based cancer diagnoses and therapy. There are both graduate- and undergraduate-level projects in the lab.
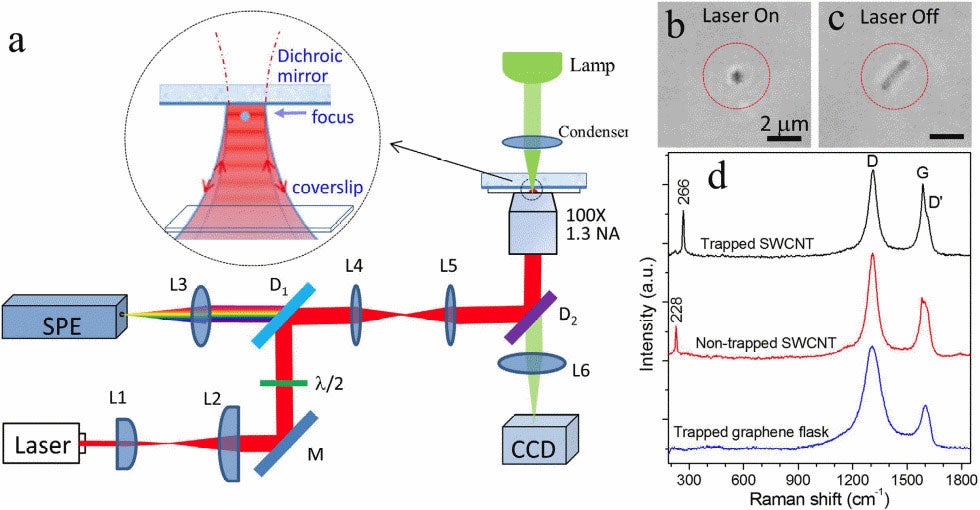
Raman tweezers is used for the stable optical trapping and characterization of nanoparticles and single cells. This figure’s source is part of a Nature collection on optical tweezers to celebrate the 2018 Nobel Prize in Physics.

(top left) Dr. Sprague reads data on an ECU research vessel. (top right) Modeling sound propagation into shallow water. (bottom left) Oscillogram and spectrogram of weakfish sound. (bottom right) Recovering the Wave Glider.
Mark Sprague studies physical and biological acoustics. He applies knowledge of sound propagation to study animals (such as fishes and whales) that make sound underwater as well as the effects of human-produced sounds on underwater animals. Projects include using the acoustic Wave Glider Blackbeard to study offshore soundscapes, using hydrophone arrays to locate sound-producers in extremely shallow oyster marshes. He also uses propagation modeling to understand the sound exposure of animals near navigational channels, models sound production in fishes, and is developing a new instrument to measure acoustic particle velocity. There are both graduate- and undergraduate-level projects in the lab.
Juan Beltran-Huarac studies the interaction of functional nanoconstructs with biological systems in the presence of super low-frequency AC magnetic fields to treat cancer. Work in the Magnetic Formulations and Bionanotechnology Laboratory uses techniques such as
- magneto-mechanical actuation,
- PCR array,
- Western blot,
- ELISA,
- immunohistology,
- flow cytometry,
- cytoviva,
- confocal microscopy,
- magnetic resonance imaging,
- relaxometry,
- low-range magnetometry,
- dynamic light scattering,
- transmission electron microscopy,
- nanoparticle tracking analysis,
- and thermal decomposition
to understand how mechanical motion of field-activated nanoconstructs induces changes in cell function and tumor microenvironments. Projects involve developing magnetic nanoformulations, determining their magneto-mechanical properties and in/extrinsic magnetic responses, tracking them within cells and tissues, and evaluating their potential (and/or in combination with drugs) to induce cell death and inhibit tumor growth. There are both graduate- and undergraduate-level projects in the lab.
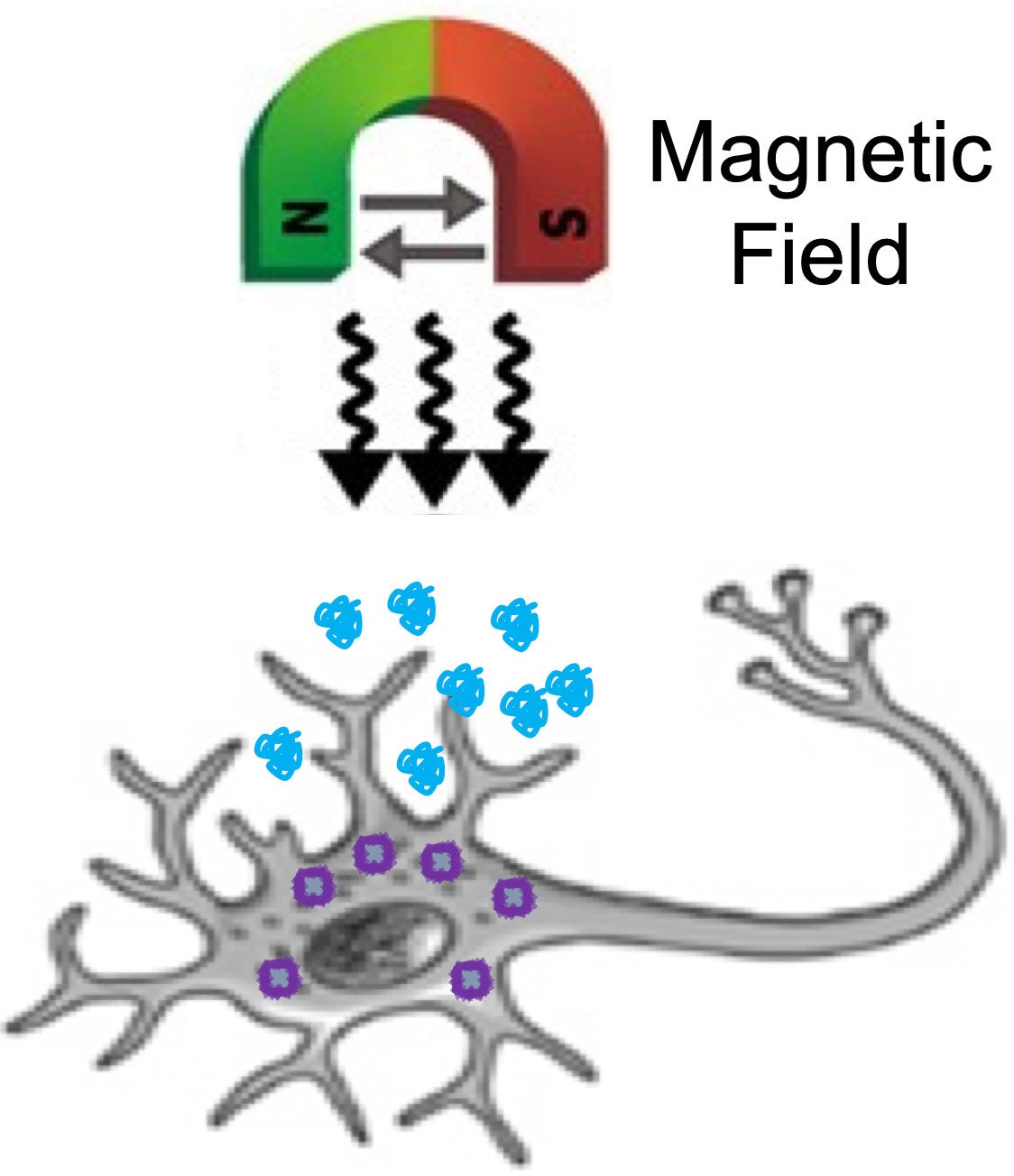
Schematic of the conceptual strategy to treat cancer via magneto-mechanic actuation.